Amorphous Material Is Best Described as
Amorphous film materials can be formed by. A glass is a solid that has been cooled too quickly to form ordered crystals.

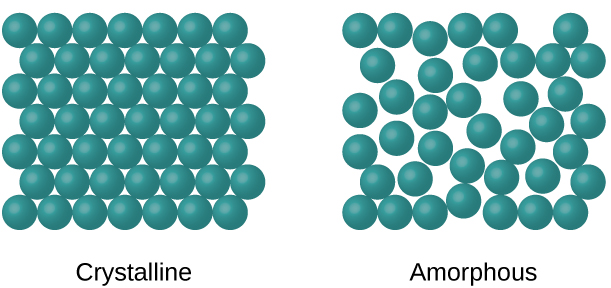
Crystalline And Amorphous Solids Explanation Differences Examples Etc
Being without definite character or nature.

. Solids that Flow Like Liquids. The Tg of an amorphous material is defined as the transition temperature between the glassy state solid-like material and the rubbery state liquid-like material. A dislocation in an.
Some examples of amorphous solids they are thermoplastic polymers thermoset polymers elastomers expandable polymers or glass. It was once thought that relatively few materials could be prepared as amorphous solids and such materials notably oxide glasses and organic polymers were called glass-forming solids. It is now known that the amorphous solid state is almost a universal property of condensable matter.
Glass Gels plastics various polymers wax thin films. An amorphous material AM has a non-crystalline structure that differs from that of its iso-chemical liquid and does. Hence they are known as isotropic materials.
It is highly rigid at room temperature but it does not have the long-range microscopic regularity of a solid crystal lattice. We distinguish at least four categories of amorphous glassy materials. I Amorphous material is best described as.
Amorphous comes from the Greek where the prefixais negation and the word morfo means form that is formless. This situation is sometimes described as short-range order and long-range disorder and is typical of all glassy materials including those which are not compounds of Si. Iii organic and inorganic thermoplastics.
Circle one a A material whose atoms are defined by at least four atoms or ions. More recent additions to the family of amorphous solids are synthetic polymers 1940s and metallic glasses 1960s. Rapid quench 105 Ks X-ray amorphous forms include any material structural coherence length is or the order of 5 basic units eg.
The terms amorphous and non-crystalline are synonymous under this definition. Having no definite form. Atoms molecules unit cells.
This paper focuses on the models of atomic arrangements in amorphous materials. Amorphous solids display several of the characteristics of a liquid. An amorphous material AM has a non-crystalline structure that differs from that of its iso-chemical liquid and does not undergo structural relaxation and the glass transition when heated.
Deposition at low temperatures where the adatoms do not have enough mobility to form a crystalline structure quenching. And iv amorphous permanent networks. This study categorizes the global Health and Safety Products.
The best way to differentiate between amorphous and crystalline materials is to measure XRD patterns. Amorphous materials cannot contain dislocations. Pyrolusite for example is probably an amorphous manganese dioxid produced by the dehydration of crystalline manganite which accounts for its fibrous structure.
Modern approach is to carry out computer simulations with prediction that can be tested by experiments. An amorphous translucent solid is called a glass An amorphous translucent solid. Preparation of amorphous solids.
In the absence of cleavage and other direct proofs of crystal- linity we must rely largely upon optical tests for transparent and translucent minerals. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Properties structure and durability.
Crystalline material always exhibit sharp diffraction peaks. 38 minutes agoAmorphous Metal Material Market Size 2022-2028 presents detailed competitive analysis including the market Share Size Future scope. Amorphous ice is most likely the most common form of water in space.
The table of representative amorphous solids. The terms amorphous and non-crystalline are synonymous under this definition. Unclassifiable an amorphous segment of society.
Just like in liquids in amorphous solids properties such as electrical conductivity thermal conductivity mechanical strength refractive index etc remain the same in all directions. There are naturally occurring amorphous materials such as obsidian mineral vitrified rocks and solidified organic materials such as amber. Shapeless an amorphous cloud mass.
Amorphous materials are those that have no detectable crystal structure. B A material whose properties t c A material that lacks the long-range order. Man made inorganic glasses date back to ancient civilisations Summeria Egypt.
D A atrial wiit cubic symmery in which ihe. Amorphous material is one In which there is no definite atomic structure and atoms exist in a random pattern just as in a liquid. Httpwww2cnrsfren1231html accessed on 23 August 2011.
Amorphous alloys are a class of metal. The earliest ideas of Bernal on the structure of liquids were followed by experiments and computer models for the packing of spheres. Deposition of a natural glassy material such as a glass composition.
Google Scholar Bernal JD. The term glassy has the same structural. Liquids Glasses crushed Crystals sometimes Meso-phases sometimes and potentially very small.
When we speak of amorphous solids we speak of a solid state of matter in which the particles that make it do not. A geometrical approach to the structure of liquids. Comprehensive Biotechnology Second Edition 2011.
The dislocation density in an amorphous material is normally less than the dislocation density in a crystalline material with the same composition. Fused silica is an example of an amorphous material or glass. The dislocation density in an amorphous material is normally greater than the dislocation density in a crystalline material with the same composition.

Amorphous Solid Definition Examples Video Lesson Transcript Study Com
Comments
Post a Comment